Introduction
Up to 72% of real-world multiple myeloma (MM) pts are considered ineligible for RCTs due to criteria such as old age, frailty, and comorbidities, limiting the generalizability of findings to the wider MM population. The US MM-6 study (NCT03173092) aimed to increase the duration of proteasome inhibitor (PI)-based therapy and improve outcomes with a tolerable safety profile in a patient (pt) cohort representative of that in routine clinical practice. Results for the fully accrued dataset have been reported previously (N=141; Yimer IMS 2022). Here we report the results of a US MM-6 subanalysis describing pt characteristics and key efficacy and safety outcomes of two groups: RCT-ineligible (RCT-i) and RCT-eligible (RCT-e).
Methods
Transplant-ineligible/delayed-transplant (≥24 months) NDMM pts with ≥stable disease after 3 cycles of parenteral V-based induction were enrolled at US community sites to receive all-oral ixazomib-lenalidomide-dexamethasone (IRd) for up to 39 cycles or until progression or toxicity (Manda CLML 2020). Pts were grouped based on whether any baseline characteristic met common RCT ineligibility criteria including but not limited to hematologic/organ dysfunction, ECOG PS >2, renal dysfunction, prior malignancies, cardiac dysfunction, and pulmonary disease. Two-year progression-free survival (PFS; primary endpoint), overall survival (OS), overall response rate (ORR), and safety were analyzed by RCT group.
Results
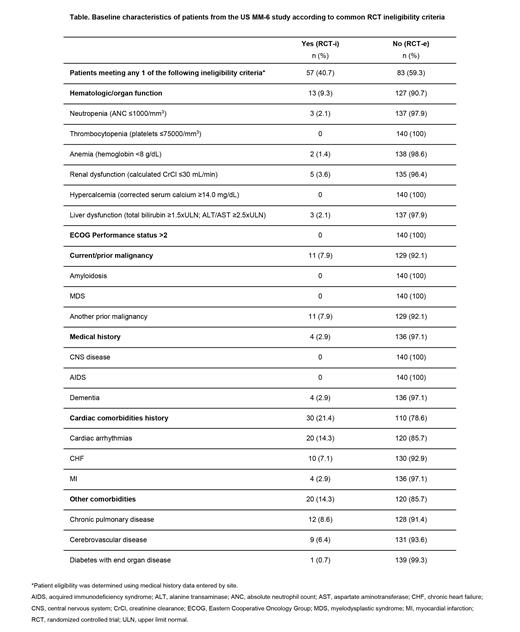
At final data cut-off (Oct 17, 2022) 140 pts were evaluable; 57 (40.7%) met ≥1 RCT ineligibility criteria and were included in the RCT-i group; 83 (59.3%) were included in the RCT-e group. The most common ineligibility criteria in the RCT-i group were cardiac arrhythmias (14.3%), chronic pulmonary disease (8.6%), and prior malignancy (7.9%) (Table). At enrollment, in the RCT-i vs RCT-e groups, median age was 73 years (range 48-90) vs 72 years (range 49-86), 54.4% vs 60.2% were male, 77.2% vs 69.9% were white. In RCT-i vs RCT-e, 24.6% vs 27.7%, 40.4% vs 42.2%, and 33.3% vs 30.1% had ISS stage I, II, and III, respectively. A total of 98.2% vs 90.4% of pts in the RCT-i vs RCT-e group had any comorbidity; 15.8% vs 10.8% had peripheral neuropathy (PN) as a comorbidity. At data cut-off, 84.2% of pts in the RCT-i group had discontinued treatment, commonly (>20% of pts) due to progressive disease (27.1%), adverse events (25.0%), and pt withdrawal (20.8%). In the RCT-e group, 75.9% had discontinued treatment, commonly due to pt withdrawal (25.4%) and adverse events (23.8%). In both RCT-i and RCT-e groups, pts received a median of 11 (range 1-39) IRd and ixazomib cycles; median duration of PI therapy was 13.6 months. After a median follow-up of 29.2 vs 24.6 months for RCT-i vs RCT-e groups, 2-year PFS rates were 68% (95% confidence interval [CI] 52-80) vs 73% (95% CI 60-82); 2-year OS rates were 90% (95% CI 78-96) vs 84% (95% CI 72-91). ORR increased from 61.4% vs 66.3% at the end of V-based induction to 80.7% vs 79.5% after iCT for RCT-i vs RCT-e groups. For the respective RCT-i and RCT-e groups, treatment-emergent adverse events (TEAEs) were observed in 98.2% and 97.6% of pts (treatment-related: 82.5% and 81.9%), and serious TEAEs in 43.9% and 44.6% (treatment-related: 12.3% and 13.3%) of pts; on-study deaths occurred in 3 pts and 1 pt. The most common any-grade TEAEs (>30% of pts in either arm) were diarrhea (RCT-i: 49.1%; RCT-e: 51.8%), fatigue (RCT-i: 38.6%; RCT-e: 30.1%), and PN (RCT-i: 31.6%; RCT-e: 25.3%). In the RCT-i and RCT-e groups, respectively, grade ≥3 TEAEs were observed in 73.7% and 65.1% of pts. The most common grade ≥3 TEAEs in RCT-i pts were pneumonia (8.8%), cellulitis, neutropenia, and hypokalemia (all 7.0%). Among RCT-e pts the most common grade ≥3 TEAEs were diarrhea (12.0%), pneumonia, anemia, and fatigue (all 4.8%). TEAEs led to dose modification/discontinuation of any of the three study drugs in the IRd regimen in 68.4%/21.1% of RCT-i pts and 65.1%/19.3% of RCT-e pts.
Conclusions
This subanalysis demonstrates the feasibility of utilizing iCT to obtain similar outcomes to RCTs in the community. Although 41% of US MM-6 pts would have been ineligible for RCTs, there were no notable differences in PFS, ORR, OS, and safety data between the two groups. These data show that iCT from V-based induction to IRd permits long-term, tolerable PI-based therapy translating into improved efficacy in community-treated pts with NDMM who are more representative of the wider MM population.
OffLabel Disclosure:
Girnius:Sanofi: Honoraria; Takeda: Honoraria, Speakers Bureau; BMS: Honoraria, Speakers Bureau; Janssen: Honoraria, Speakers Bureau; Beigene: Speakers Bureau; Amgen: Speakers Bureau. Richter:Astra Zeneca: Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy; Bristol-Meyers-Squibb: Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees. Bogard:Takeda Oncology: Current Employment. Yimer:Abbvie, Amgen, AstraZeneca, BeiGene USA, Inc., GlaxoSmithKline, Janssen Biotech, Karyopharm Therapeutics, Takeda: Speakers Bureau. Manda:Genmab: Current holder of stock options in a privately-held company. Lyons:Incyte Corporation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; McKesson: Other: Leadership; Texas Oncology: Current holder of stock options in a privately-held company; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Exact Sciences: Research Funding; Pfizer: Research Funding; Astellas Pharma: Research Funding; Lessen: Consultancy, Membership on an entity's Board of Directors or advisory committees. Noga:Takeda Oncology: Current Employment, Current equity holder in publicly-traded company. Rifkin:Sanofi: Membership on an entity's Board of Directors or advisory committees; Coherus: Membership on an entity's Board of Directors or advisory committees; Fresenius-Kabi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; McKesson - Biosimilar Medical Director: Current Employment, Current equity holder in publicly-traded company.
Real-world evaluation of long-term proteasome inhibition with ixazomib in combination with lenalidomide and dexamethasone for the treatment of newly diagnosed multiple myeloma in non-transplant patients with stable disease after 3 cycles of a bortezomib-based induction.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal